
 |
Problem: H2A is a diprotic acid. Ka1 = 4.7 E-6, Ka2 = 2.7 E-11. A 20.00 mL sample of 0.100 M Na2A solution is titrated with 0.200 M HCl. What will the pH of the solution be after additions of 0, 1, 5, 9.5, 10, 12, 20, and 23 mL of the acid? |
|||||||||||||||||||
Solution: Hey! Wait a second! This isn't fair! Titrating a base with an acid is different from what we're used to! Yep, we know, and Mr. McAfoos knows too, which is why this is fair game for the death test. But before you stab yourself in the heart with your mechanical pencil, take a look at the problem. Everything's the same. Here are the reactions going on in solution: |
|||||||||||||||||||
|
|||||||||||||||||||
Now let's find the equilibrium constants: |
|||||||||||||||||||
|
|||||||||||||||||||
Note: Here's the logic behind the equilibrium constant for reaction number 5:
|
|||||||||||||||||||
Ba da bing, ba da boom. Now, the equivalence point and the max buffer points: |
|||||||||||||||||||
MBVB = MAVA (0.100 M)(20.00 mL) = (0.200)(equivalence point) Equivalence point 1 = 10 mL Equivalence point 2 = 2 * 10 mL = 20 mL Max buffer point 1 = (0 + 10)/2 = 5 mL Max buffer point 2 = (10 + 20)/2 = 15 mL |
|||||||||||||||||||
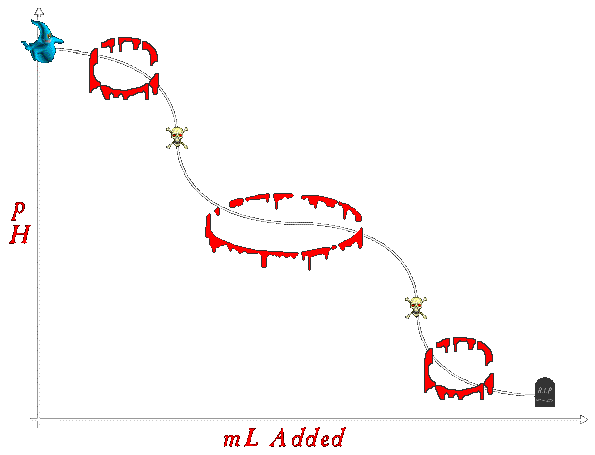
Okay, now that we know all the important points, it's time to draw a spiffy titration curve. I don't know, maybe one like this: (Click on each section of the graph for the lowdown on what reactions are happening, what the dominant reaction is, and the math for the relevant points) |
 --Starting Point --Starting Point
|
 --Equivalence Point --Equivalence Point
|
 --End Point --End Point
|
 --Buffer Region --Buffer Region
|
|
|
mL |
V (L) |
MBVB |
MAVA |
HA¯ |
[OH¯] |
pH |
|---|---|---|---|---|---|---|
| 0 | 0.02 | (0.1)(0.02) = 0.002 | 0 | --- | 0 = X2/(0.1 - X) - 3.7 E-4 X = 5.9 E-3 | pH=11.8 |
mL |
V (L) |
MBVB |
MAVA |
HA¯ |
pOH |
pH |
|---|---|---|---|---|---|---|
| *** Use Henderson/Hasselbach *** | ||||||
| 1 | 0.021 | 0.002 - 0.0002 = 0.0018 | (0.001)(0.2) = 0.0002 | 0.0002 | pOH = pKb1 - log(0.0018/0.0002) = 2.5 | pH=11.5 |
| *** Max Buffer Point *** | ||||||
| 5 | --- | --- | --- | --- | pOH = pKb1 = 3.4 | pH=10.6 |
| *** We're out of the H/H range--back to equilibrium! *** | ||||||
| 9.5 | 0.0295 | 0.002 - 0.0019 = 0.0001 | (0.0095)(0.2) = 0.0019 | 0.0019 | 0 = X(0.0019/0.0295 + X)/(0.0001/0.0295 - X) [OH¯] = X = 1.9 E-5 pOH = 4.7 | pH=9.3 |
mL |
V (L) |
MBVB |
MAVA |
HA¯ |
H2A2- |
pH |
|---|---|---|---|---|---|---|
| *** Use Henderson/Hasselbach *** | ||||||
| 12 | 0.032 | 0.002 | (0.012)(0.2) = 0.0024 - 0.002 = 0.0004 | 0.002 - 0.0004 = 0.0016 | 0.0004 | pH = pKa1 - log(0.0004/0.0016) = 5.9 |
mL |
V (L) |
MBVB |
MAVA |
HA¯ |
H2A2- |
pH |
|---|---|---|---|---|---|---|
| 20 | 0.04 | 0.002 | (0.02)(0.2) = 0.004 - 0.002 = 0.002 | 0.002 - 0.002 = 0 | 0.002 | 0 = X2/(0.002/0.04 - X) - 4.7 E-6 [H3O+] = X = 4.8 E-4 pH = 3.3 |
mL |
V (L) |
MBVB |
MAVA |
HA¯ |
H2A2- |
pH |
|---|---|---|---|---|---|---|
| 23 | 0.043 | 0.002 | (0.023)(0.2) = 0.0046 - 0.002 = 0.0026 - 0.002 = 0.0006 | 0.002 - 0.002 = 0 | 0.002 | 0 = X(0.0006/0.043 + X)/ (0.002/0.043 - X) - 4.7 E-6 |
Most excellent! You're through the toughest part of the Death Test. Why don't you go grab Rufus and roll on down to Hydrolysis? |


|